Scope of Regulations
Dermal filler treatments have become increasingly popular in recent years, offering individuals the opportunity to enhance their appearance and address signs of aging. However, it’s crucial for anyone considering these treatments to understand the regulatory framework governing their administration in the UK.
Types of Fillers Regulated
The scope of regulations surrounding dermal filler treatments in the UK is comprehensive, aiming to ensure patient safety and the quality of procedures. These regulations primarily focus on the products used, the practitioners administering them, and the facilities where treatments are performed.
In the UK, various types of fillers are regulated, including hyaluronic acid fillers, calcium hydroxylapatite fillers, poly-L-lactic acid (PLLA) fillers, and collagen stimulators. Each type of filler has specific requirements for manufacturing, labeling, and safety testing.
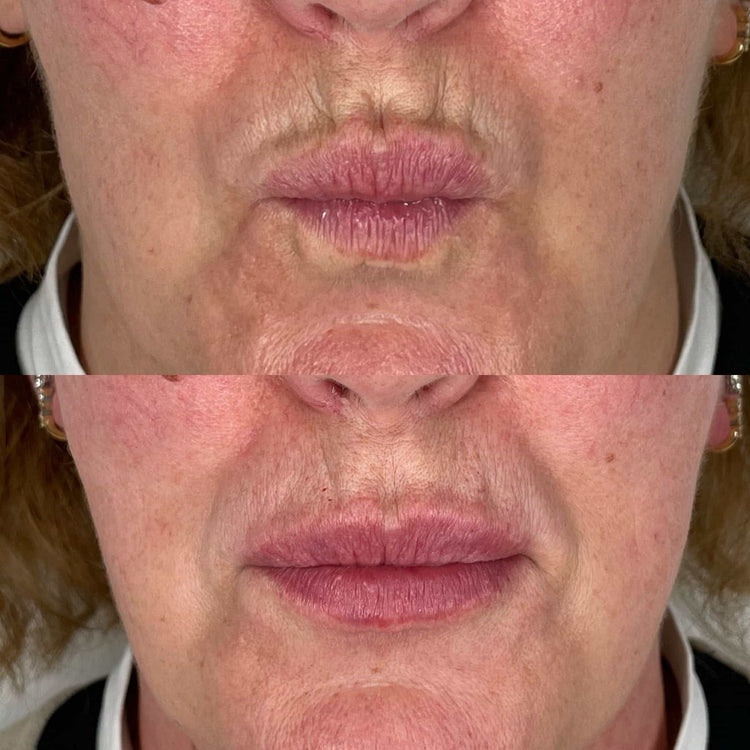
Licensing and Qualifications of Practitioners
Licensing and qualifications are crucial aspects of the regulatory framework governing dermal filler treatments in the UK. To administer dermal fillers legally, practitioners must possess appropriate qualifications and be registered with a relevant regulatory body.
The most commonly recognized qualification for administering dermal fillers is a Level 7 Diploma in Aesthetic Medicine. This diploma provides comprehensive training in facial anatomy, injection techniques, and the safe use of dermal fillers. Practitioners who hold this qualification are authorized to administer a wide range of dermal filler treatments.
Safety and Standards
Dermal filler treatments have become increasingly popular in recent years, offering individuals the opportunity to enhance their appearance and address signs of aging.
The scope of regulations surrounding dermal filler treatments in the UK is comprehensive, aiming to ensure patient safety and the quality of procedures. These regulations primarily focus on the products used, the practitioners administering them, and the facilities where treatments are performed.
Product Approval and Safety Testing
In the UK, various types of fillers are regulated, including hyaluronic acid fillers, calcium hydroxylapatite fillers, poly-L-lactic acid (PLLA) fillers, and collagen stimulators. Each type of filler has specific requirements for manufacturing, labeling, and safety testing.
To ensure patient safety, these products undergo rigorous safety testing before being approved for use in the UK. This testing involves a multi-stage process that assesses the filler’s biocompatibility, efficacy, and potential risks.
The Medicines and Healthcare Products Regulatory Agency (MHRA) is the primary body responsible for regulating medical devices and pharmaceuticals in the UK, including dermal fillers.
They establish and enforce standards for product approval, safety testing, and labeling. The MHRA’s role is critical in safeguarding patient health and maintaining public confidence in dermal filler treatments.
Training and Competency Requirements
Licensing and qualifications are crucial aspects of the regulatory framework governing dermal filler treatments in the UK. To administer dermal fillers legally, practitioners must possess appropriate qualifications and be registered with a relevant regulatory body.
The most commonly recognized qualification for administering dermal fillers is a Level 7 Diploma in Aesthetic Medicine. This diploma provides comprehensive training in facial anatomy, injection techniques, and the safe use of dermal fillers. Practitioners who hold this qualification are authorized to administer a wide range of dermal filler treatments.
To ensure patient safety, these products undergo rigorous safety testing before being approved for use in the UK. This testing involves a multi-stage process that assesses the filler’s biocompatibility, efficacy, and potential risks.
The Medicines and Healthcare Products Regulatory Agency (MHRA) is the primary body responsible for regulating medical devices and pharmaceuticals in the UK, including dermal fillers.
They establish and enforce standards for product approval, safety testing, and labeling. The MHRA’s role is critical in safeguarding patient health and maintaining public confidence in dermal filler treatments.
Infection Control Measures
Safety and standards are paramount in the realm of dermal filler treatments. The UK has implemented stringent regulations to ensure patient well-being and maintain the quality of procedures.
These regulations encompass various aspects, including product approval, practitioner qualifications, and facility standards.
The Medicines and Healthcare Products Regulatory Agency (MHRA) plays a pivotal role in overseeing these regulations.
They rigorously assess dermal fillers for safety and efficacy before authorizing their use in the UK.
Practitioners administering dermal fillers must possess appropriate qualifications and be registered with relevant regulatory bodies.
A Level 7 Diploma in Aesthetic Medicine is a widely recognized qualification for this purpose, providing comprehensive training in facial anatomy, injection techniques, and safe filler practices.
Adverse Effects and Reporting Procedures
Understanding the potential adverse effects of dermal filler treatments and the procedures for reporting them is essential for both patients and practitioners. Adverse effects can range from mild, such as swelling or bruising, to more serious complications like infection or vascular occlusion. It is crucial that anyone experiencing any unusual or concerning symptoms following a dermal filler treatment promptly seeks medical attention.
Recognizable Side Effects
Understanding the potential adverse effects of dermal filler treatments and the procedures for reporting them is essential for both patients and practitioners. Adverse effects can range from mild, such as swelling or bruising, to more serious complications like infection or vascular occlusion. It is crucial that anyone experiencing any unusual or concerning symptoms following a dermal filler treatment promptly seeks medical attention.
Recognizable side effects of dermal fillers include redness, swelling, tenderness, and itching at the injection site. These are typically temporary and resolve within a few days to weeks. More serious side effects, although rare, can occur and include allergic reactions, infection, vascular occlusion (blockage of blood vessels), and granulomas (lumps under the skin).
If you experience any adverse effects following a dermal filler treatment, it is important to contact your practitioner immediately. They will be able to assess your symptoms and provide appropriate treatment.
In case of severe or life-threatening reactions, seek emergency medical attention. The Medicines and Healthcare products Regulatory Agency (MHRA) also encourages patients to report any suspected adverse drug reactions related to dermal fillers through their website or yellow card scheme.
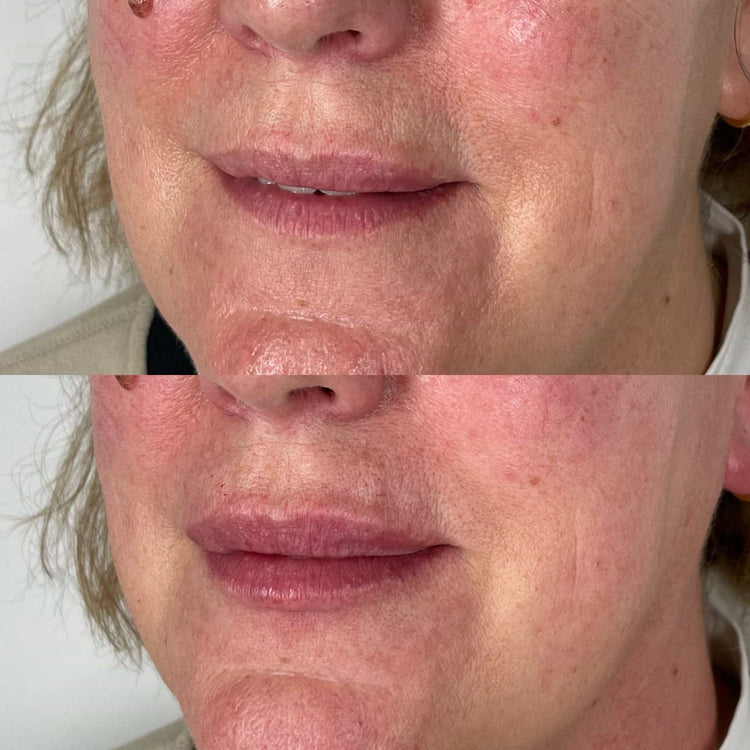
Reporting Mechanisms for Complications
Understanding the potential adverse effects of dermal filler treatments and the procedures for reporting them is essential for both patients and practitioners. Adverse effects can range from mild, such as swelling or bruising, to more serious complications like infection or vascular occlusion. It is crucial that anyone experiencing any unusual or concerning symptoms following a dermal filler treatment promptly seeks medical attention.
Recognizable side effects of dermal fillers include redness, swelling, tenderness, and itching at the injection site. These are typically temporary and resolve within a few days to weeks. More serious side effects, although rare, can occur and include allergic reactions, infection, vascular occlusion (blockage of blood vessels), and granulomas (lumps under the skin).
If you experience any adverse effects following a dermal filler treatment, it is important to contact your practitioner immediately. They will be able to assess your symptoms and provide appropriate treatment.
In case of severe or life-threatening reactions, seek emergency medical attention. The Medicines and Healthcare products Regulatory Agency (MHRA) also encourages patients to report any suspected adverse drug reactions related to dermal fillers through their website or yellow card scheme.
Post-Treatment Care Instructions
Understanding the potential adverse effects of dermal filler treatments and the procedures for reporting them is essential for both patients and practitioners. Adverse effects can range from mild, such as swelling or bruising, to more serious complications like infection or vascular occlusion. It is crucial that anyone experiencing any unusual or concerning symptoms following a dermal filler treatment promptly seeks medical attention.
- Recognizable side effects of dermal fillers include redness, swelling, tenderness, and itching at the injection site. These are typically temporary and resolve within a few days to weeks.
- More serious side effects, although rare, can occur and include allergic reactions, infection, vascular occlusion (blockage of blood vessels), and granulomas (lumps under the skin).
If you experience any adverse effects following a dermal filler treatment, it is important to contact your practitioner immediately. They will be able to assess your symptoms and provide appropriate treatment.
In case of severe or life-threatening reactions, seek emergency medical attention. The Medicines and Healthcare products Regulatory Agency (MHRA) also encourages patients to report any suspected adverse drug reactions related to dermal fillers through their website or yellow card scheme.
Legal Consequences of Non-Compliance
Non-compliance with UK regulations surrounding dermal filler treatments can result in a range of legal consequences. These consequences may include fines, suspension of practitioner licenses, or even criminal charges in cases of serious harm to patients.
Penalties for Unqualified Practitioners
Practitioners who administer dermal fillers without the necessary qualifications risk severe penalties. The General Medical Council (GMC) and other regulatory bodies have strict guidelines regarding practitioner competence.
Operating without proper authorization or engaging in procedures beyond their scope of practice can lead to disciplinary action, including fines, suspension of licenses, or even revocation of registration.
Patients who choose unqualified practitioners face significant risks, as they may not receive safe and effective treatment. This could result in complications, adverse effects, and long-term health issues.
It is crucial for both practitioners and patients to adhere to UK regulations surrounding dermal filler treatments.
Product Liability Issues
Non-compliance with UK regulations surrounding dermal filler treatments can result in a range of legal consequences. These consequences may include fines, suspension of practitioner licenses, or even criminal charges in cases of serious harm to patients.
Practitioners who administer dermal fillers without the necessary qualifications risk severe penalties. The General Medical Council (GMC) and other regulatory bodies have strict guidelines regarding practitioner competence.
Operating without proper authorization or engaging in procedures beyond their scope of practice can lead to disciplinary action, including fines, suspension of licenses, or even revocation of registration.
Patients who choose unqualified practitioners face significant risks, as they may not receive safe and effective treatment. This could result in complications, adverse effects, and long-term health issues.
Product liability issues can also arise in cases where dermal fillers cause harm to patients due to defects in the product itself or improper handling by the practitioner.
Manufacturers of dermal fillers are legally obligated to ensure their products are safe and effective for their intended use. If a defect in a filler leads to patient harm, the manufacturer could be held liable.
Similarly, practitioners can be held liable if they administer dermal fillers negligently, causing harm to patients. This includes failing to obtain informed consent, using improper techniques, or failing to adequately assess and manage potential risks.
Consumer Protection Laws
Non-compliance with UK regulations surrounding dermal filler treatments can result in a range of legal consequences. These consequences may include fines, suspension of practitioner licenses, or even criminal charges in cases of serious harm to patients.
Practitioners who administer dermal fillers without the necessary qualifications risk severe penalties. The General Medical Council (GMC) and other regulatory bodies have strict guidelines regarding practitioner competence.
Operating without proper authorization or engaging in procedures beyond their scope of practice can lead to disciplinary action, including fines, suspension of licenses, or even revocation of registration.
Patients who choose unqualified practitioners face significant risks, as they may not receive safe and effective treatment. This could result in complications, adverse effects, and long-term health issues.
Product liability issues can also arise in cases where dermal fillers cause harm to patients due to defects in the product itself or improper handling by the practitioner.
Manufacturers of dermal fillers are legally obligated to ensure their products are safe and effective for their intended use. If a defect in a filler leads to patient harm, the manufacturer could be held liable.
Similarly, practitioners can be held liable if they administer dermal fillers negligently, causing harm to patients. This includes failing to obtain informed consent, using improper techniques, or failing to adequately assess and manage potential risks.
Learn more about the benefits of dermal fillers with Dr. Laura Geige at It’s Me & You Clinic
- Why The Craftsman Series Vape Is A Top Choice For Oil Enthusiasts - January 25, 2026
- Why Does My Lip Filler Not Last - January 24, 2026
- Why Am I Losing So Much Hair At My Temples? - January 21, 2026
